|
|
Solubility Testing |
|
|
|
Solubility Testing |
|

Introduction:
Most people think that Peeps are primarily sugar. We all know that sugar dissolves in water. This means that Peeps should dissolve in water, right?? Wrong!
To the left you may witness the paradigm-shattering discovery which led to the following battery of tests. As you can see, this Peep is floating quite comfortably in a beaker of water. In fact, we have seen Peeps float for hours on end with no noticeable difficulty.
Of course, floating in room temperature water is one thing, and floating in hot water is another. Following our room temperature tests, we tried heating up the water to see if that would increase Peep solubility. (Note, hot water looks just like cold water. Please look again at the image above and pretend that if you were to touch the beaker you would burn yourself. Thanks.)
Alas, Peeps are as resistant to hot water as they are cold. Disappointed that we were unable to dissolve Peeps in pure water, we knew we'd have to explore this topic further.
Materials & Methods:
We assembled four Peeps, four 600mL beakers, and four solutions. The solutions are (as seen below from left to right): water, acetone, sulfuric acid, and sodium hydroxide.

One Peep subject was gently plunked into each solution and we recorded our observations over the next hour. For details on the chemical properties of each solution and a close-up photo of the results of each of these tests, please click on the solution of choice below:

[Water] [Acetone] [Sulfuric Acid] [Sodium Hydroxide]
Results Summary:
Essentially, we found that Peeps are not soluble in water (a polar liquid), acetone (a non-polar liquid), sulfuric acid (a strong acid), or sodium hydroxide (a strong base). In fact, with the exception of the acetone turning purple as some of the sugar did dissolve off the surface of the Peep, there was no noticeable reaction of any kind. For specific descriptions of the solutions tested and their effects, click on the links above.
Preliminary Conclusion:
Lesser scientists may have been discouraged by such an apparent failure, however we looked at this as a challenge. Perhaps Peeps have a protective outer coating which is resistant to all the above solutions. To evaluate this theory, known as the Natural Outer Resistance Against Dissolving (NORAD) defense system, follow-up experiment one was carried out.
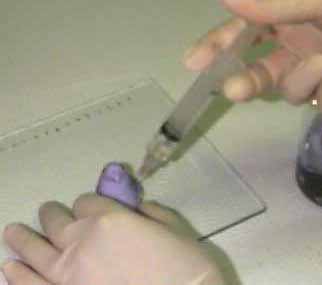
Follow-up Experiment Number One:
To test NORAD, we needed some way to get our solutions past the potential defense system. A 30cc syringe seemed a natural choice.
We were running low on test subjects at this point, so we decided to test only the solution with which we expected the greatest reaction. Sulfuric acid was chosen as the test condition.
At this point, I should admit that we really didn't expect that the Peep would dissolve in the sulfuric acid, but we were hoping that the acid would react with some of the sugar in the Peep causing rapid oxidation and turning the Peep into a large mass of blackened goo.
Alas, we were disappointed as the subcutaneous injection produced no greater reaction than immersion in the acid. It seems that the NORAD defense hypothesis had been refuted. Soon the NORAD hypothesis was rejected, and the "Nastier Solvent" approach was adopted. Follow-up experiment number two describes our progress.
Follow-up Experiment Two: Nastier Solvent
If Peeps aren't composed of sugar, what are they? Protein (gelatin) seemed the only other choice. Phenol seemed, then, to be our best choice of solvent (phenol is used in labs specifically to dissolve proteins). To give you an idea for how icky this stuff really is, here is what the Merck index says about Phenol:
Ingestion of even small amounts may cause nausea, vomiting, circulatory collapse, tachypnea, paralysis, convulsions, coma, greenish or smoky-colored urine, necrosis of mouth and G.I. tract, icterus, death from respiratory failure, sometimes from cardiac arrest. Average fatal dose is 15g, but death from as little as one gram has been reported. Fatal poisoning may also occur by skin absorption...
Results:
The results are best illustrated by the following series of photos.
|
Time = 0 The Peep was prepared for immersion in approximately 100 mL of Phenol. |
|
|
|
Time = 5 Min A change was evident almost immediately, as the Peep appeared to absorb the Phenol (note the dark area near the base of the Peep).
|
|
Time = 35 Min Our persistence was rewarded as we noticed the outer portions of the Peep dissolving away.
|
|
|
|
Time = 50 Min Very little of our subject remained at this point. The general Peepish shape is still visible to the left. |
|
Time = 65 Min Quite unexpected (and disturbing) results became evident after a little more than one hour of solubility testing. As can be seen to the right, although the Peep body was dissolved by phenol, the eyes were not. In fact, Peep eyes were insoluble in all the solutions which were tested.
|
|
Conclusion:
Given enough time, the proper resources, and access to some really toxic stuff, one can probably dissolve just about anything except Peep eyes.